| Agricultural Nutrients |
|
![]() |

COWIN, the Chinese Leading producer of calcium chloride, supplies products suitable for use in the agricultural industry.
All of these products contain 100% water soluble calcium, which gives our customers a tremendous advantage over those using much less soluble calcium sources such as gypsum and limestone.
The products are used:
- As a nutrient to supply calcium for crops, alone or in a blend of other nutrients
- As a soil amendment to reduce sodium levels, improve soil tilth and flocculate the soil, and
- As a post harvest dip to increase the shelf life of fruits and vegetables.
- As a manure additive to reduce ammonia evaporation by decreasing the pH
- As an acidifying salt for reducing DCAB in feed to prevent milk fever
- As an ingredient in drinking water for chickens, where calcium supports the development of bones and shell and to reduce heat stress by improving the mineral/salt balance.
Calcium as a Nutrient
Calcium is necessary to the growth and development of all plant life. Only nitrogen and potassium are required in larger amounts by plants.
The following will give you a brief overview of calcium's important role in the growth of plants.
Calcium is considered a secondary plant nutrient. Only nitrogen and potassium are required in larger amounts by plants.
The Functions of Calcium
- Every plant needs calcium to grow.
- Once fixed, calcium is not mobile in the plant. It is an important constituent of cell walls and can only be supplied in the xylem sap. Thus, if the plant runs out of a supply of calcium, it cannot remobilize calcium from older tissues.
- If transpiration is reduced for any reason, the calcium supply to growing tissues will rapidly become inadequate.
Without adequate amounts of calcium, plants experience a variety of problems.
The Benefits of Calcium
Calcium plays a very important role in plant growth and nutrition, as well as in cell wall deposition.
The primary roles of calcium:
- As a soil amendment, calcium helps to maintain chemical balance in the soil, reduces soil salinity, and improves water penetration.
- Calcium plays a critical metabolic role in carbohydrate removal.
- Calcium neutralizes cell acids.
The role of calcium in plants must not be overlooked.
Factors Affecting Calcium Availability
Calcium is found in many minerals in soil, but is relatively insoluble in this state. Calcium is not considered a leachable nutrient. Many soils will contain high levels of insoluble calcium such as calcium carbonate, but crops grown in these soils will often show a calcium deficiency.
High levels of other cations such as magnesium, ammonium, iron, aluminum and especially potassium, will reduce the calcium uptake in some crops. A common misconception is that if the pH is high, adequate calcium is present. This is not always true.
Calcium Deficiency
Calcium deficiency symptoms in crops are often called physiological disorders.
Symptoms of calcium deficiency:
- Necrosis at the tips and margins of young leaves,
- Bulb and fruit abnormalities,
- Deformation of affected leaves,
- Highly branched, short, brown root systems,
- Severe, stunted growth, and
- General chlorosis.
It must be remembered that these problems are caused by an inadequate supply of calcium to the affected tissues. These deficiencies can occur even when the soil appears to have an adequate presence of calcium.
Toxicity Issues
For all practical purposes, calcium is not considered to be toxic to plants. Although rare, excess calcium levels in the soil can reduce a plant uptake of other nutrients such as phosphorus, potassium, magnesium, boron, copper, iron, or zinc, resulting in deficiencies of these nutrients.
Using Calcium in a Fertility Program
When calcium is needed, it is not necessary to apply a material such as limestone that will affect the pH level in the soil. Hi-Cal� fertilizer can supply 100 percent of the recommended soluble calcium and will not affect soil pH levels. In today抯 crop production, this effect is most desirable because the soluble calcium can be applied through an irrigation system when needed and in the amounts that are needed. Because calcium does not relocate in the plant, a soluble source of calcium applied throughout the growing season is preferred, especially in vegetables and other fast growing crops.
Go to the Top
| Animal Feed |
|
![]() |

Dry calcium chloride is used in various animal feed mixes. In dairy cow feeding, calcium chloride is being used increasingly. Giving calcium chloride to transition cows aids in reducing the incidence of milk fever and other subclinical hypocalcemia disorders. The calcium chloride acts as a cation-anion balance, allowing the proper absorption of calcium.
COWIN's solid calcium chloride products have the following elemental analysis:
| Component |
Minimum |
| Calcium (Ca) |
33.9% |
| Chloride (Cl) |
61.0% |
COWIN's dihydrate calcium chloride products have the following elemental analysis:
| Component |
Minimum |
| Calcium (Ca) |
27.8% |
| Chloride (Cl) |
50.0% |
Elemental Ca and Cl data is derived from CaCl2 based upon an assay of 94-97 percent calcium chloride, with a factor for trace amounts of MgCl2 and NaCl of less than one percent.
Go to the Top
| De-Icing |
|
![]() |

Calcium chloride is the most powerful of all common de-icers used for melting ice and snow on sidewalks, driveways, parking lots, and streets. The exothermic (heat releasing) reaction when solid calcium chloride goes into solution makes it the de-icer of choice. No other product melts more ice, and melts ice faster, than calcium chloride.
Calcium chloride has the lowest freezing point of all the typical de-icers including sodium chloride, magnesium chloride, potassium chloride, and urea.
Calcium chloride is an essential part of any winter road maintenance program and will provide a safe surface for both traffic and pedestrians.
Both liquid and solid calcium chloride can be used for de-icing. The selection of a product will depend on the local conditions and available equipment. |
|
|
|
|
|
|
|
|
Go to the Top
| Distilleries |
|
![]() |

Tartaric acid is naturally Grapesoccurring in grapes and other fruits and plants. In the wine and grappa industries, it can be found in the solid residues that remain after the grape juice has been produced.
Crude tartaric acid can be recovered from the following sources:
- The press cakes from grape juice [i.e., unfermented (marcs) or partly fermented (pomace)] are boiled with water, and any alcohol that is present is distilled off. The hot mash is settled, decanted and the clear liquor is cooled to crystallize. The recovered high-test crude cream of tartar (vinaccia) has an 85-90% tartaric acid content.
- Lees, which are the dried sediments in the wine fermentation vats, consist of yeast cells, pectinous substances, and tartars. Their content of tartaric acid equivalents ranges from 16% to 40%.
- The crystalline crust (argols) formed in the vats in the secondary fermentation period contains more than 40% tartaric acid.
Calcium tartrate is a sparingly soluble salt that can be precipitated by adding calcium chloride to these residues.
Go to the Top
| Dust Binding |
|
![]() |

Calcium chloride has unique properties that make it ideal for maintaining unpaved roads and fortifying road bases for new construction. Calcium chloride is both hygroscopic (draws moisture from the air) and deliquescent (resists evaporation and stays in solution). Calcium chloride's ability to regulate moisture on road surfaces is the key to building and maintaining roads that last.
Utilizing calcium chloride will heavily decrease the loss of material from the road surface from the effects of traffic and wind. This decrease will provide economical as well as environmental and health benefits. Maintenance costs will be reduced, and the displacement of small airborne particles will be minimized, helping to alleviate this recognized health issue.
Both liquid and solid calcium chloride can be used for dust binding. The selection of a product will depend on the distribution equipment available and other local factors. |
|
|
|
|
|
|
|
|
Go to the Top
| Chemical Heating |
|
![]() |

One effective and creative use of the energy in calcium chloride is in self heating containers. Water and anhydrous calcium chloride can be incorporated in anHot advanced container and then brought into contact with each other by pressing a button, which causes the seal between the two materials to rupture. The heat evolved in the dissolution of the calcium chloride can then be used to heat a beverage such as coffee or tea.
When calcium chloride is dissolved in water, heat is evolved since calcium chloride has a negative heat of solution. The amount of energy available depends on the concentration of crystal water; a completely water free (anhydrous) calcium chloride contains the most energy. The table below gives the theoretical maximum energy that is available from one gram of product with varying concentrations of calcium chloride.
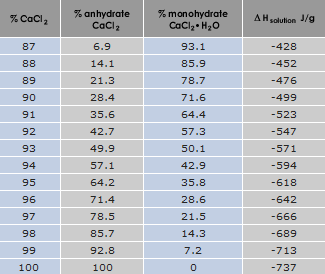
The reason for the rapid drop in available energy as the concentration of calcium chloride decreases can be found in the heat of solution for pure anhydrous CaCl2 and the monohydrate as shown below.
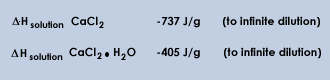
Go to the Top
| Concrete Acceleration |
|
![]() |

The primary benefit of calcium chloride in concrete is an accelerated rate of hydration for cement. This significantly reduces setting time to facilitate rapid attainment of early strength and easier protection of freshly placed concrete in cold weather.
Manufacturers of ready mixed concrete have used calcium chloride for over 50 years to give their contracting customers the performance they want and the durable product they require.
Ready mixed concrete containing calcium chloride offers significant economic benefits to concrete contractors by reducing costs and improving their overall performance and profits.
Cool Weather
Anytime the temperature drops below 20°C, the setting of ready mixed concrete slows down. This effect is most significant in the 0°C to 10°C temperature range. The rapid setting offered by the addition of calcium chloride to ready mixed concrete allows concrete contractors to continue to work effectively into the fall and winter months.
In cool and cold weather concreting applications, calcium chloride can be used to reduce setting times by as much as two-thirds. A two percent admixture of calcium chloride at 10°C will improve setting times to that attainable at 20°C without calcium chloride.
This rapid setting can be critical when there is a chance that temperatures may drop below freezing. Calcium chloride accelerates the rate of hydration of freshly placed concrete and lowers its free water content faster.
This allows concrete to escape potential freeze damage in a matter of days, as opposed to the weeks it can take normally. It also minimizes the costly precautions required to protect freshly placed concrete from serious freeze damage.
High Early Strength
In applications where time is of the essence such as foundations and emergency patching of pavement, the addition of calcium chloride to ready mixed concrete can reduce the waiting period before surfaces can bear loads. |
|
|
|
|
|
|
|
|
|
Go to the Top
| Dehumidification |
|
![]() |

Dehumidification of air is an important process in both industrial and domestic applications.
Domestic applications of calcium chloride are as dehumidifiers for closets, cupboards, cars, boats and mobile homes. In many cases, the humidity in these types of enclosed spaces is too high, leading to accelerated micro-biological activity. This growth of mold and bacteria often leads to unwanted odors and may also degrade materials such as textiles, wood, paper and leather. If the air humidity is very high, condensation of water can occur, which can damage many materials and goods by staining or initiating corrosion. By using a calcium chloride dehumidifier, these problems can often be mitigated or completely solved.
In industrial applications, the aim is the same as given above—to reduce humidity in order to protect goods, machinery and electronics. For example, absorption of moisture in transport containers during sea transport requires TETRA's solid calcium chloride as a cost effective and efficient moisture absorbing chemical. |
|
|
|
|
|
Go to the Top
| Enzyme Production |
|
![]() |
Enzymes are protein structures that act as extremely efficient catalysts. Enzymes are used in many industrial and pharmaceutical applications. Industrial applications include, e.g., using enzymes for detergents. Calcium chloride can be utilised in different applications within the enzyme industry, e.g., as a method to facilitate separation of the enzyme from other cell structures after fermentation. Some enzymes also require a calcium ion, Ca2+, as co-factor that will retain the three-dimensional structure of the protein. In some cases, a highly soluble source of calcium is required as a nutrient in fermentation processes. |
|
|
|
|
|
|
|
|
Go to the Top
| Food Processing |
|
![]() |

| Calcium chloride has applications in many areas in the food industry, since calcium is an essential component of plant structures and is necessary in animal and human nutrition. COWINs food grade products are the highest quality in the industry and meet the requirements of the relevant FCC, EC and FAO standards.
Fruits and Vegetables: It is used to increase the firmness of fruits and vegetables, preventing breakup in processing and cooking.
- Cheese Manufacturing: It is used to increase the size and strength of the curds.
- Beverage Manufacturing: The bottling industry uses it to remove sodium alkalinity from water used in soft drink and beer formulation. Water is then remineralized to desired levels, assuring uniform taste regardless of processing location.
- Novelty Ice Cream: Calcium chloride is also used as a refrigerant in molds used to manufacture novelty ice cream and frozen dessert products.
Calcium chloride is also used as a meat tenderizer and a flavor enhancer. COWIN's food grade products are Kosher certified
. |
|
|
|
|
|
|
|
|
Go to the Top
| Fluoride Removal |
|
![]() |

| The treatment of fluoride bearing effluents is an area of increasing concern, as fluoride content in drinking water has become a part of the public and regulatory agenda. Industrial operations, such as petroleum refineries, aluminum smelters, and semi-conductor production facilities, generate effluents that are high in fluoride content and require treatment prior to their discharge into public streams. Calcium chloride can be used effectively in the removal of fluoride.
For more information, please see the attached paper entitled, Treatment of Aqueous Effluents for Fluoride Removal, which outlines using calcium chloride as an effective method of fluoride removal.
Calcium chloride can also be used to remove other undesired organisms from industrial wastewaters, e.g., soluble phosphates can be precipitated as calcium phosphate and sulphate as calcium sulphate (gypsum). |
|
|
|
|
|
|
|
|
|
Go to the Top
| Gas and Solvent Drying |
|
![]() |

Refineries and petrochemical plants requiring a method to remove dissolved and free water from hydrocarbon streams can utilise calcium chloride pellets that significantly outperform other means of moisture removal.
COWIN supplies calcium chloride pellets that are effective in drying the following and similar products:

Solid calcium chloride can, due to its affinity for water, also be used for removing water from certain oils and organic solvents. |
|
|
|
|
|
Go to the Top
| Mining Industry |
|
![]() |

| Freeze Conditioning of Minerals:
Minerals, particularly coal, as they are normally transported and handled, contain substantial amounts of moisture. If shipped or stored in cold weather of sufficient duration and intensity, moist minerals tend to freeze. Frozen minerals create severe handling problems during the winter months. For example, bottom-dumped railcars that unload in a few minutes in the summer months may take hours to unload when frozen. The use of freeze conditioning agents (FCA) to ease unloading and handling of minerals during the winter months has become an accepted practice by the mineral industry.
FCAs have two main benefits. They function as a freeze point depressant, and also weaken the crystal structure of the ice formed, making the ice more friable. Calcium chloride, being an excellent additive to weaken the crystal structure of the ice and superior as a freeze point depressant, is widely used in the mineral industry as a freeze conditioning agent.
The amount of calcium chloride required depends on the expected weather condition and the moisture content of the minerals. For coal, with the moisture content of coal being about 10 wt%, the amount required could be 1-2 litres per ton of coal.
Dust Control in Mining Operations:
Both underground and open cut mining operations are regulated for the level of dust emission in the working environment. Since April 2, 1998, according to the Mine Safety and Health Administration (MSHA) in the U.S.A. a noncompliance determination is based on the results of single full-shift dust samples taken by the inspectors. MSHA requires mine operators to take corrective action to lower the concentration of respirable dust whenever a full-shift measurement by an MSHA inspector indicates non-compliance.
Calcium chloride, being hygroscopic and deliquescent, when spread on the haulage roads minimizes the dust emission. It is also very cost effective.
Calcium chloride is also used in mining operations for the stabilization of the unpaved haulage roads, as its application improves the strength of the road surface. Apart from keeping the dust level down, its use also substantially reduces the overall road maintenance cost. |
|
|
|
|
|
|
|
|
|
Go to the Top
| Oil and Gas Drilling |
|
![]() |

COWIN offers calcium chloride products that are used in:
- Completion and workover fluids,
- Basic drilling fluids,
- Oil based mud/inverted drilling fluids, drill-in fluids, and horizontal drilling fluids,
- Clear water flocculation applications, and
- Cementing applications.
Calcium chloride is used as a clear brine single salt fluid in densities ranging from 1006 kg/m3 to 1390 kg/m3 (8.4 to 11.6 lb/gal). The divalent calcium ion (Ca+2) inhibits clay swelling, dispersion and migration. Calcium chloride brines are one of the most economical brines used in oilfield completion and workover operations.
COWIN has been developing and delivering high quality, innovative clear brine fluids (CBFs) and related products and services to the oil and gas industry for well over two decades.
With seven North American and three European calcium chloride production facilities and a significant global marketing presence, COWIN is the leading worldwide supplier of calcium chloride.
For more information on our clear brine fluids and additives designed for use in the oil and gas industry, please visit the Fluids and Filtration section of the COWIN Industry Limited Web site. |
|
|
|
|
|
|
Go to the Top
| Tire Weighting |
|
![]() |

Calcium chloride, when dissolved and used as a liquid for tire ballast, will provide a number of benefits. It will increase traction, drawbar pull, tire and tractor life, and will also help you conserve fuel.
Your local implement or tire dealer will assist with tire hydraflation. A 28% solution of calcium chloride will give a freezing point of below -35degree. |
Mixing Guidelines
There are a number of ways you can prepare 100 kg of 28% calcium chloride solution:
- mix 64 litres of cold tap water and 36 kg 77% flakes, or
- mix 70 litres of cold tap water and 30 kg 94% granules, or
- mix 22 litres of cold tap water and 58 litres (77 kg) of 36% solution.
|
|
|
|
|
|
|
|
Go to the Top
| Our Plants & Equipments |
|
![]() |

|